Optimization and production of clinical grade MSC
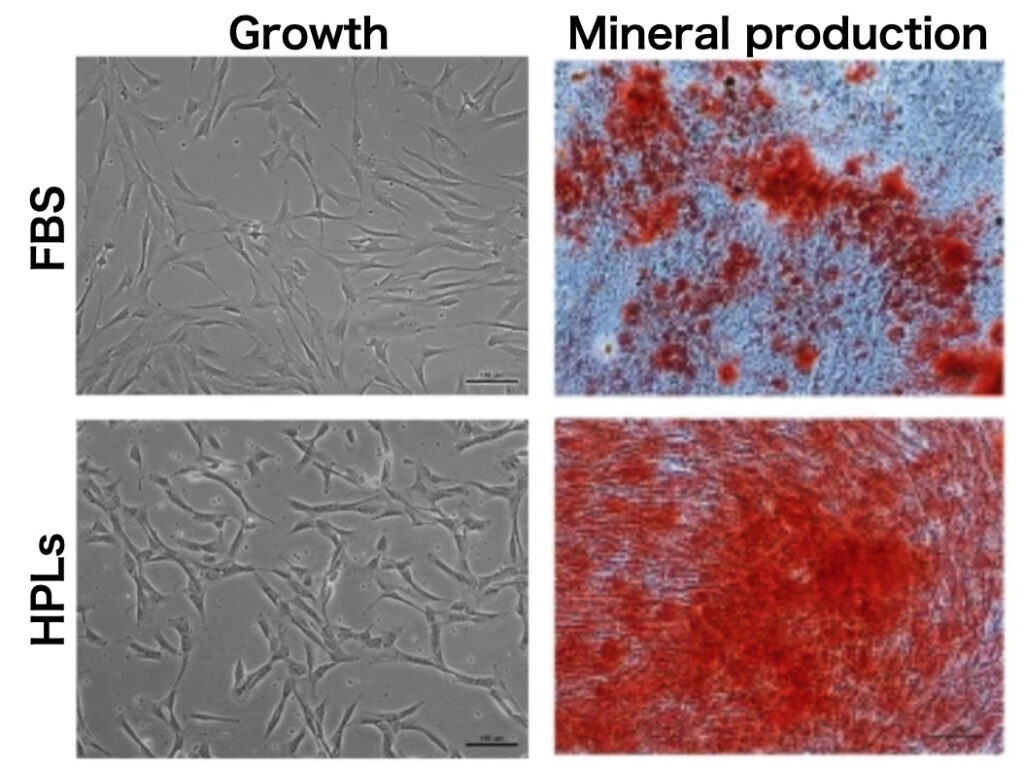
Generally, animal serum (i.e., Fatal Bovine Serum: FBS) is often utilised in cell culture as a source of nutrient for optimal cell growth.
However, using animal products often hinder the production of clinical grade cell source for transplantation in accordance of cGMP regulation. Therefore, this project mainly aims at optimizing xeon-free cell culture conditions to produce clinical grade mesenchymal stem cells (MSC), particularly by using human platelet lysate (HPLs).
Human platelet-derivatives, prepared from pooled platelet concentrates which are no longer suitable for transfusion, represent optimal supplements to replace animal serum, due to the nature and concentrations of growth factors released by platelets which are favorable for stem cell expansion.
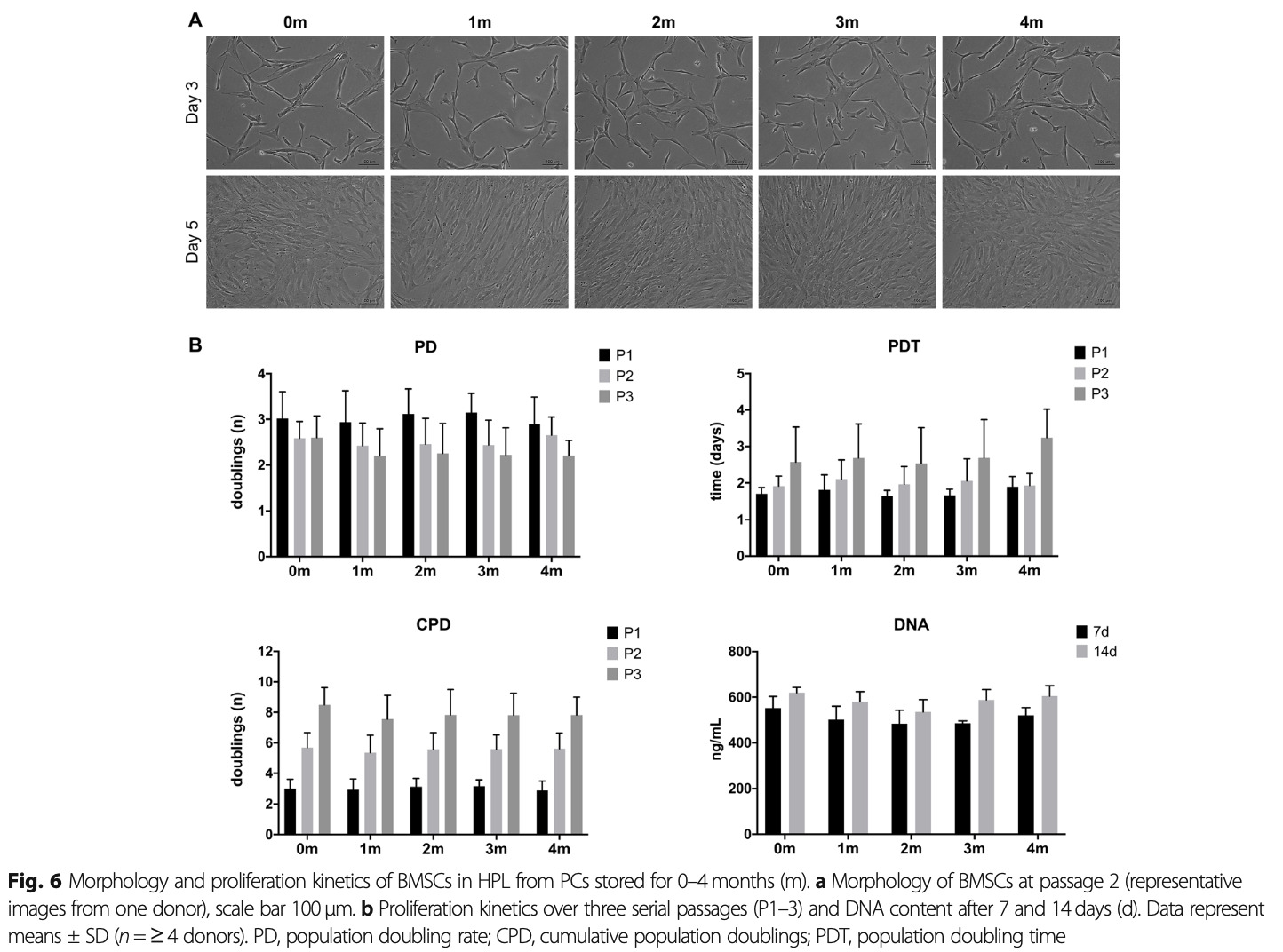
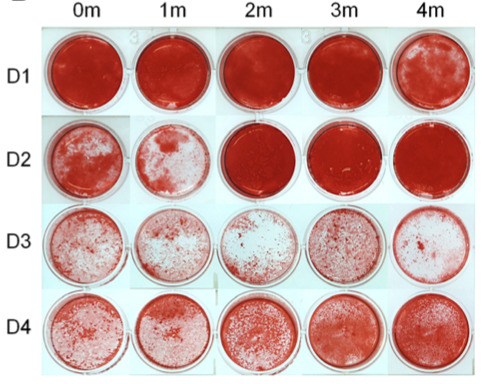
A substantial effort has been placed in the present project to optimize HPLs for stem cell expansion using outdated platelet concentrates, focusing specifically on the storage time.
Our data show that HPLs produced from outdated platelet concentrates stored for up to 4 months efficiently supported the proliferation and osteogenic differentiation of MSC. These findings may facilitate the standardization and scaling-up of HPL for bone tissue engineering applications.
A PhD thesis titled “Xeno-free 3D Culture of Mesenchymal Stromal Cells for Bone Tissue Engineering” was successfully defended in December 2020 (Shanbhag S).
The project is linked to the Ex Vivo Facility and Centre for Regenerative Medicine at Haukeland University Hospital.